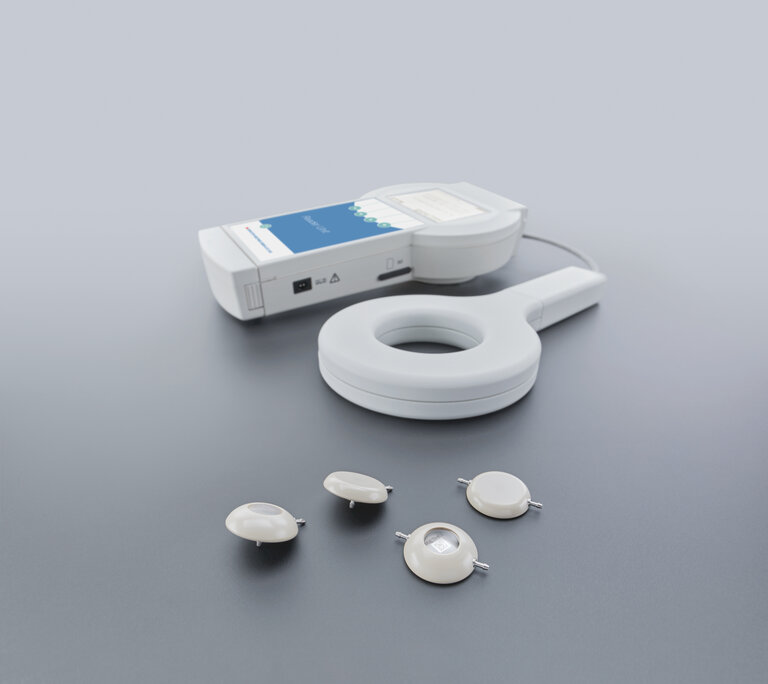
M.scio®
FEATURES
- Innovative, easy-to-use telemetric ICP sensor1,2
- Multifunctional use for diagnosis and therapy support2,3
- Improvement of clinical symptoms in up to 75% of shunt patients through effective therapy adjustments with M.scio support2,4
- Savings potential of 69% in treatment costs for hydrocephalus patients4
- Optimized patient management through reduction in the number of shunt revisions, diagnostic procedures and hospitalizations4-6
- Stable long-term implant with high life time7,8
- Display of detailed pressure curves due to high sampling rate
of 44 Hz2,5 - Puncturability of the silicone membrane (M.scio dome variants)3,5
- Reliable readings due to low drift of < 2 mmHg / 4 years7
- MR conditional up to 3 Tesla9
SENSOR TECHNOLOGY
FOR TELEMETRIC MEASUREMENT OF INTRACRANIAL PRESSURE
Hydrocephalus and subarachnoid hemorrhages may be associated with a life-threatening increase in intracranial pressure (ICP). Determining the ICP is an important requirement for proper application of ICP-lowering measures.10 To date, there is no way to adequately determine intracranial pressures based on symptoms or imaging methods alone.11,12
In addition to measurement for initial diagnosis, it may be necessary to monitor intracranial pressure repeatedly over a period of years to ensure the desired therapeutic outcome. The information gathered can help prevent time-consuming and costly examinations and hospitalizations, thus improving patient well-being.2,4,5
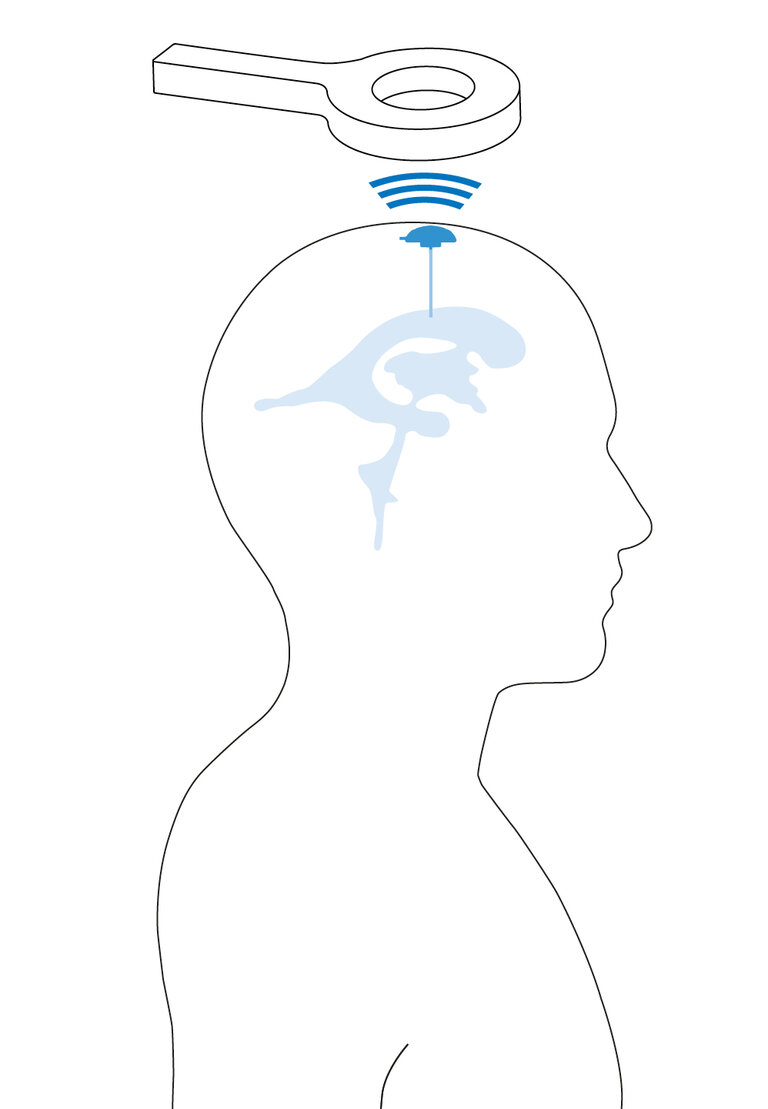
The M.scio is a long-term implant for simple, non-invasive telemetric measurement of intracranial pressure.
From diagnosis to therapy
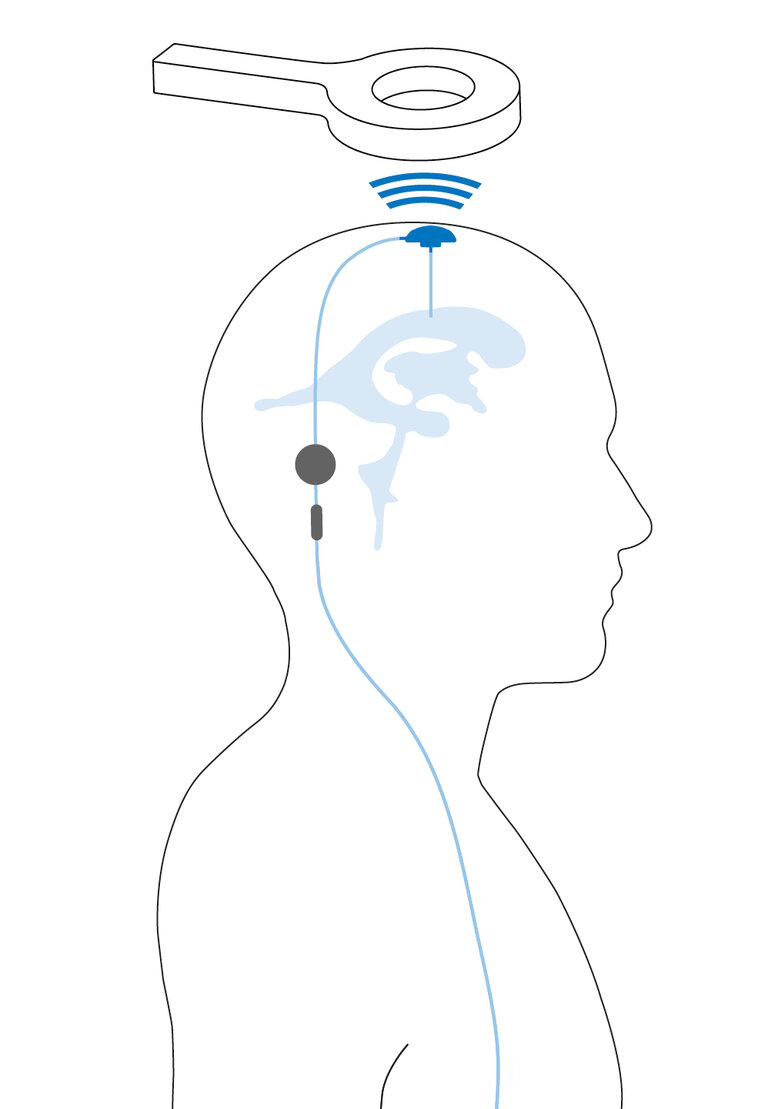
It can be implanted to determine intracranial pressure for diagnostic purposes.3 Moreover, the M.scio can also be integrated into a shunt to determine the ICP in the shunt.2-4 This allows conclusions to be drawn about the functionality of the shunt system and may help to steer treatment in a positive direction.2
M.scio®
FUNCTIONALITY
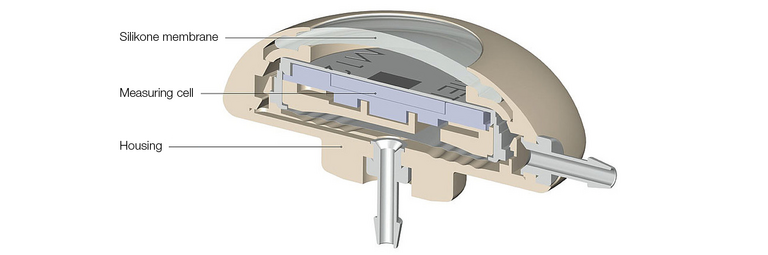
Pressure is measured via a measuring cell located inside the M.scio. The electronics for pressure measurement are protected by the titanium housing of the measuring cell against external, negative influences that could impair its function - for a high functional reliability and a long service life.
The housing of the M.scio is made of the biocompatible polymer PEEK. The materials used are of the highest-quality and have been tested and standardized for use as implant materials. The catheters are made of silicone and are latex-free.
The M.scio is available in four variants: dome-angled, flat-angled, dome-inline, and flat-inline. Both “dome” variants fulfill the characteristics of a conventional reservoir in addition to intracranial pressure measurement. This means they allow therapeutic pressure relief through withdrawal of cerebrospinal fluid (CSF), diagnostic sampling of CSF, administration of fluids, and verification of pressure readings.
Reader Unit Set
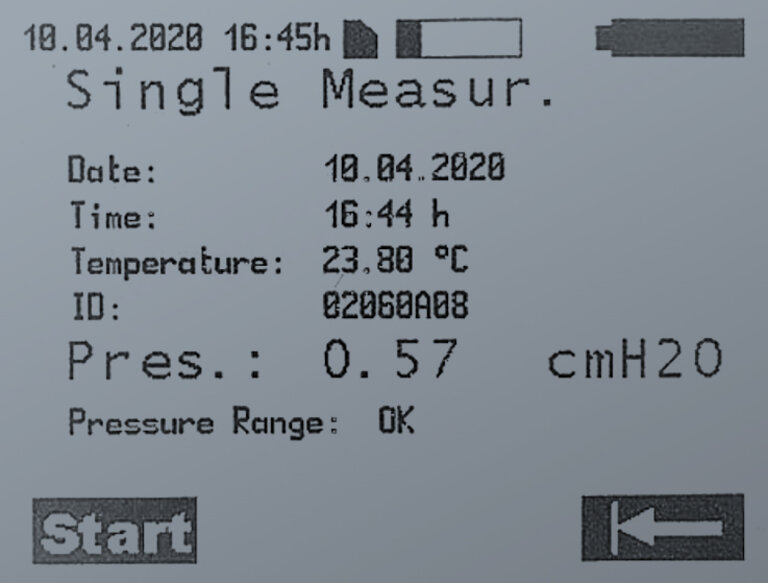
SINGLE MEASUREMENT
With the single measurement, the pressure value measured at a point is displayed as a single measured value.
The measuring unit of the pressure value can be selected in the settings.
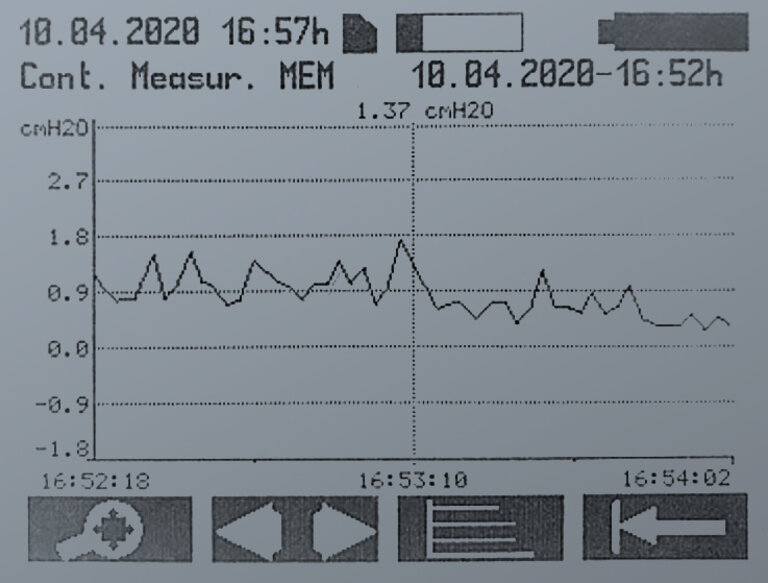
CONTINUOUS MEASUREMENT
During the continuous measurement, sequential single measurements are performed and the recorded measured values are displayed as a curve. The interval between the single measurements can be adjusted in the settings in the range from 1 to 300 seconds.
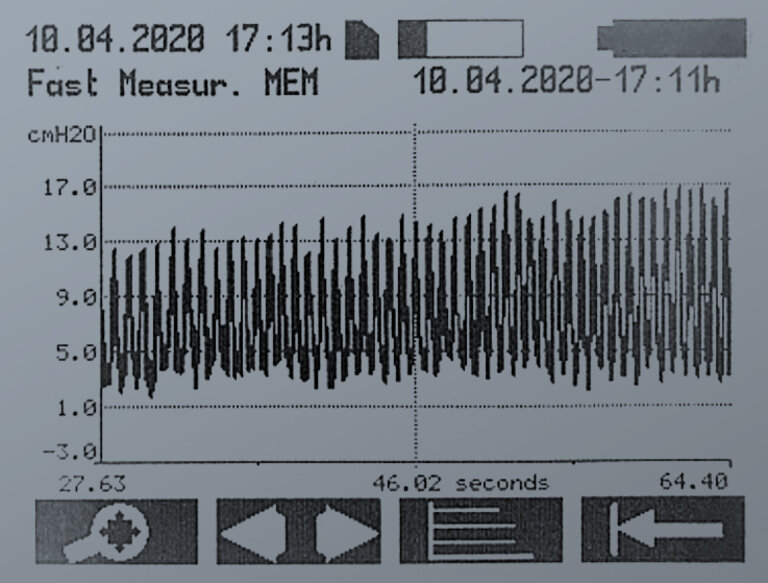
FAST MEASUREMENT
With the fast measurement, sequential single measurements are recorded at a high sampling rate (44 measurements per second) and displayed as a curve.
Quality Reference
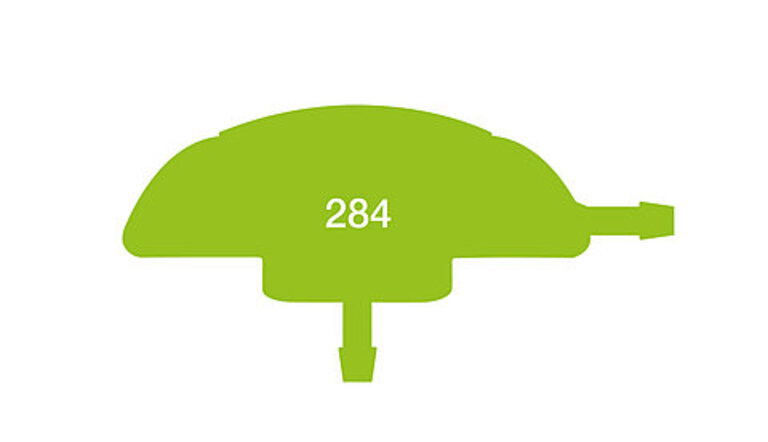
RELEVANT DATA SIZE:
HIGH FEEDBACK RATE
This survey was conducted in 2019 and contains feedback on a total of 284 implanted pressure sensors.
HIGH SURVIVAL RATE:
STABLE LONG-TERM IMPLANT
95% (270) of the pressure sensors were still implanted in the patients at the time of the survey - without malfunction, many of them for more than three years.
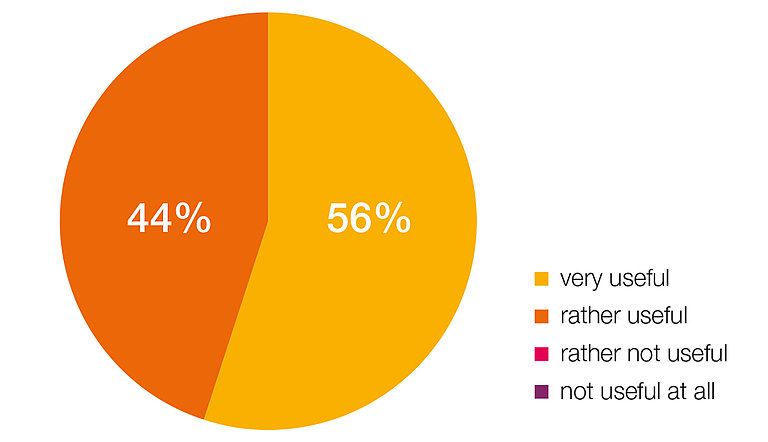
HIGH USER SATISFACTION:
HELPFUL PRODUCT
100% of the users of the pressure sensors have evaluated it as a helpful product and an advantageous addition in the treatment of hydrocephalus.
DO YOU HAVE ANY QUESTIONS ABOUT THE PRODUCT?
WE ARE THERE FOR YOU
![[Translate to English:] Zwei Marketing Mitrabeiter*innen blättern durch M.scio Prospekte von MIETHKE](/fileadmin/_processed_/f/8/csm_SalesTeam_a8fc997cce.png)
FOR OUR SALES EXPERTS
We are there for you!
Dear sales & shunt experts all over the world,
our toolbox is big. If you need something to make MIETHKE shunts easier to understand or have new ideas, please get in touch with us. We look forward to exchanging ideas and - who knows - maybe what you are looking for is already there and we can easily make it available to you. Or we can develop something with you that could also be very helpful for other markets.
Your MIETHKE Marketing Team

OUR PARTNERSHIP
WITH B. BRAUN
B. Braun and MIETHKE - Together for a better life with hydrocephalus
We have a long and intensive partnership with B. Braun in the field of neurosurgery. We are driven by a common vision: to improve the lives of hydrocephalus patients around the world with innovative solutions.
Our partnership is an exciting combination of B. Braun's nearly 180 years of expertise as one of the world's leading medical device and pharmaceutical companies and our agility as an innovative company and technology leader in gravitation-based shunt technology.
Our strong partner in neurosurgery:
References
(1) Ertl P, Hermann EJ, Heissler HE, et al. Telemetric Intracranial Pressure Recording via a Shunt System Integrated Sensor: A Safety and Feasibility Study. J Neurol Surg A Cent Eur Neurosurg 2017;78(6):572-5.
(2) Antes S, Stadie A, Muller S, et al. Intracranial Pressure-Guided Shunt Valve Adjustments with the Miethke Sensor Reservoir. World Neurosurg 2018;109:e642-e50.
(3) Pennacchietti, V.; Prinz, V.; Schaumann, A.; Finger, T.; Schulz, M.; Thomale, U. W. Single center experiences with telemetric intracranial pressure measurements in patients with CSF circulation disturbances. Acta neurochirurgica 2020[cited 2022 Apr 28];162(10):2487-97. Available from: https://doi.org/10.1007/s00701-020-04421-7
(4) Bjornson, A.; Henderson, D.; Lawrence, E.; McMullan, J.; Ushewokunze, S. The Sensor Reservoir-does it change management? Acta neurochirurgica 2021[cited 2022 Apr 28];163(4):1087-95. Available from: https://doi.org/10.1007/s00701-021-04729-y
(5) Norager NH, Lilja-Cyron A, Hansen TS, et al. Deciding on Appropriate Telemetric Intracranial Pressure Monitoring System. World Neurosurg 2019;126:564-9.
(6) Thompson S, Thorne L, Toma A, et al. Telemetric monitoring of ICP within a shunt system. A single centre experience including the first in vivo comparison versus conventional intraparenchymal monitoring. Fluids Barriers CNS. 2017;14(Suppl 1):A63.
(7) MIETHKE Bench-Test
(8) PMCF-Survey 2019
(9) Shellock FG, Knebel J, Prat AD. Evaluation of MRI issues for a new neurological implant, the Sensor Reservoir. Magn Reson Imaging 2013;31(7):1245-50.
(10) Huttner H et al.,IntrakraniellerDruck (ICP), S1-Leitlinie,2018 in: Deutsche Gesellschaft für Neurologie(Hrsg.),Leitlinien für Diagnostik und Therapie in der Neurologie. Available from: www.dgn.org/leitlinien [cited 2022 Apr 28].
(11) Evensen KB, Eide PK. Measuring intracranial pressure by invasive, less invasive or non-invasive means: limitations and avenues for improvement. Fluids Barriers CNS. 2020[cited 2022 Apr 28];17 (1):34. Available from: https://doi.org/10.1186/s12987-020-00195-3
(12) Le Roux P. Intracranial Pressure Monitoring and Intracranial Pressure Monitoring and Management. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Boca Raton (FL): CRC Press/Taylor and Francis Group; 2016. [cited 2022 Apr 28] Available from: https://www.ncbi.nlm.nih.gov/books/NBK326713/






![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/4/1/csm_csm_MJ_Art1_teaser_973099fd31.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/5/7/csm_csm_MJ_Art2_teaser_102e3c3a7e.jpg)







